
Compiled by Valentina Todorova1 and Tsenka Konsulova2
1Institute of Oceanology, Bulgarian Academy of Sciences, PO Box 152, 9000 Varna, Bulgaria
2Institute of Oceanology, Bulgarian Academy of Sciences, PO Box 152, 9000 Varna, Bulgaria
October 2005

Compiled by Valentina Todorova1 and Tsenka Konsulova2
1Institute of Oceanology, Bulgarian Academy of Sciences, PO Box 152, 9000 Varna, Bulgaria
2Institute of Oceanology, Bulgarian Academy of Sciences, PO Box 152, 9000 Varna, Bulgaria
October 2005
Table of contents
Table of content
1.Introduction
2.Purpose
3.Sampling strategy.
4.Logistics.
4.1Site location
4.2 Sampling equipment
4.3Vessel
4.4Positioning equipment
4.5Personnel
5.Ship-board routines
5.1Grab deployment
5.2Sieving.
5.3Fixation
5.4Staining.
5.5Labelling
5.6Sample registering
6.Laboratory routines
6.1Sorting and taxonomic identification.
6.2Abundance determination.
6.3Biomass determination.
7.Data reporting.
8.Recommendations for Quality Assurance
8.1Equipment calibration
8.2Training.
8.3Repeatability of site positioning.
8.4Quality and quantity of the sample.
8.5Accuracy and traceability of sample numbering and registration.
8.6Accuracy of sample sorting and taxon identification.
8.7Accuracy of data compilation
8.8In-house Quality Assurance
9.Quality Control routines
9.1Extraction efficiency total taxa target.
9.2Extraction efficiency total individuals target.
9.3Total wet weight biomass target.
9.4Bray-Curtis comparison
10.Data analysis and metrics
11.Acknowledgements.
12.References.
Annex1.SAMPLE RECORD - MACROZOOBENTHOS
Annex2.DATA REPORT SHEET - MACROZOOBENTHOS
Annex3.List of taxonomic literature to be employed for the identification of macrozoobenthic species in the Black Sea.
Annex4.Provisional check list of macrozoobenthic Polychaeta, Crustacea and Mollusca encountered in the Black Sea and Azov Sea.
1. Introduction
This guideline has been adapted from established benthic grab sampling methods described in a range of documents the most important of which are Davies et al. (2001), Gray et al. (1992), Holme and McIntyre (1984), ISO 16665: 2005 (E), Kucheruk, 2004, the Manual for Marine Monitoring in the COMBINE Programme of HELCOM (2003), Rees et al (1991), Rumohr (1990), Rumohr (1999) and UK NMMP Green Book (2003). Further consideration has been given to quality assurance from other texts including Rees (2004), ICES (2004) and ICES (2005).
The aim of these recommendations is to standardise the methods used by different scientists in the riparian Black Sea countries for sampling and treatment of macrozoobenthos in order to increase the comparability of results for different areas and to enable detection of large-scale changes in the system that would not otherwise be detected by scientists or groups working independently of each other.
Although this manual contains some information on reporting metrics (Section 10), no recommendations are provided on which environmental status or diversity indices should be used for reporting purposes as part of the Black Sea Integrated Monitoring and Assessment Programme (BSIMAP). Further research/comparative work is required to evaluate which existing zoobenthos metrics best indicate the environmental status of the Black Sea or, indeed, to determine whether the development of Black Sea-specific metric is required. The primary objective of this manual is, therefore, to permit the collection and storage of robust macrozoobenthos raw data for subsequent reporting using whatever metrics are ultimately selected.
The manual provides detailed procedures for quantitative sampling and analysing soft sediment macrozoobenthos. Soft bottoms are defined as those with sediments ranging from mud to, and including, sand. The zoobenthos comprise animals living in the sediment (infauna), on the sediments (epifauna) or in close association with the seabed. Conventionally, these organisms are sub-divided on the basis of size (Table 1, from McIntyre, 1978). Size categories, though somewhat arbitrary, identify the major functional groups of organisms, each of which requires different approaches to sampling and analysis. Macrofauna is defined as animals retained on a 0.5 - 1mm sieve.
Table 1. Size categorization of zoobenthos (from McIntyre, 1978).
|
Category |
Size |
Biological features |
Sampling techniques |
Taxonomic position |
|
Microbenthos |
Pass finest sieves |
High rates of respiration and reproduction |
Plating and culturing. Cores of < 2 cm diam. |
Most Protozoa |
|
Meiobenthos |
Pass 0.5-1 mm sieves |
Medium respi-ration rates Two or more generations per year |
Cores of 2 10cm diam. |
Large Protozoa Small Metazoa |
|
Macrobenthos |
Retained on 0.5-1 mm sieves |
Low respiration rates. Two or less generations per year. Mostly infauna |
Grab sampling at least about 0.1m2 |
Medium-sized Metazoa |
|
Megabenthos |
Handpicked from samples |
As above, mostly epifauna |
Towed gear, trawls, dredge |
Large Metazoa |
Analysis of macrofaunal communities in soft-bottom sediments is an integral part of marine environmental assessment. The use of macrozoobenthic communities for estimation of the extent of environmental impact has been an enduring approach due to the following advantages of the mactofauna as an indicator of the environmental conditions:
long (compared to plankton) life spans of species, which therefore integrate environmental change over time;
sedentary or sessile mode of life, therefore organisms are not able to escape stress and integrate the environmental quality in a given area;
relatively easy to sample quantitatively;
relatively easy taxonomic identification and available taxonomic keys for most groups;
well-documented and predictive response to a number of environmental stressors (thus, community changes can be interpreted with a degree of confidence).
2. Purpose
The specific objectives to be accomplished by sampling and analysing benthic macrofauna depend on the general goal of the study. According to the Manual for Marine Monitoring in the COMBINE Programme of HELCOM (2003) in eutrophication assessment studies benthic communities are examined for the following purposes:
to monitor the spatial variability in species composition, abundance and biomass within the maritime area resulting from anthropogenic nutrient inputs;
to monitor temporal trends in species composition, abundance and biomass within the maritime area (at a timescale of years) in order to assess whether changes can be related to temporal trends in nutrient inputs;
to support the development and implementation of a common procedure for the identification of the status of the benthic communities;
to understand the relationship between nutrient concentrations and temporal trends in species/community characteristics.
The following basic attributes can be met by using benthic grab sampling in studies aimed at assessing species and habitat diversity:
establish the benthic community composition and measure the species richness within and between habitats;
establish the species which are present at a site/habitat, including their abundance and biomass within statistical limits;
measure the abundance of key species (rare, sensitive, declining, representative) in habitats;
determine the distribution of different habitats and the associated communities (biotopes);
identify rare, fragile, representative or rich biotopes at a site;
ground-truth mapped areas (established by video or acoustic ground discrimination techniques, e.g. side-scan sonar) occupied by biotopes.
3. Sampling strategy
A strategy is defined as an elaborate and systematic plan of action designed to achieve a particular goal. The monitoring strategy will define what is to be determined (measured), where, in which media, at which time and frequency, as well as its required quality. The strategy should also include information on the final use of data, including data analysis, compilations, statistical calculations, and evaluations.
Some general considerations regarding macrozoobenthos monitoring strategy include:
Establishment of the baseline community structure and the natural variability. This involves review of literature on the benthic biota and supporting habitat. If the existing information is insufficient, then an initial spatially extensive baseline survey is necessary to describe the distribution of the benthos, identify spatial patterns and to relate this to habitat type.
Sampling habitats and sites. Characteristic habitats and substrates typical of the whole monitoring area must be sampled. In general soft-sediment sea beds are more at risk from the consequences of nutrient enrichment and pollution, being depositional environments than are hard bottoms (including gravel), being usually high-energy environments, therefore soft bottom macrozoobenthic communities are usually targeted by monitoring studies for the assessment of the ecological status of coastal and transitional water.
Number of sampling stations. The number of stations is governed by spatial heterogeneity at the sea bed, predicted dispersal pathways of pollutants, and resource limitations (time, money, laboratory facilities, staff). Sample points must be spread out over the extent of the habitat studied to ensure an adequate consideration of spatial variation. It cannot be assumed that one point is representative of the habitat as a whole.
Number of replicate samples. Replicate sampling is recommended to allow statistical comparisons between stations in space and/or time. The sufficient number of replicate samples depends on the natural variability of benthic community qualitative and quantitative attributes.
Timing and temporal scales. Sampling time should avoid recruitment periods, since the temporary presence of many newly settled juveniles may lead to misinterpretation of the quantitative trends in adult populations. The meteorological conditions are also of importance for selecting the optimum time for field work at sea. Annual sampling at the same time each year is considered as adequate for monitoring of point-source discharges (Rees et all., 1991). However, severe effects of eutrophication with seasonal manifestation such as hypoxia/anoxia may dictate a higher sampling frequency. If the sampling frequency is twice per year, than sampling should take place in late winter/early spring (March/April) to establish the stable community conditions and in late summer/autumn (August/September) with a view to detecting the possible effects of nutrient enrichment (such as hypoxia) on the macrozoobenthos.
Establishment of reference conditions. The ecological status of benthic macrofauna is assessed by measuring the deviation of key benthic community attributes from reference values expected under non-degraded conditions in similar habitat types. Therefore a control reference site is required for each test site when measuring anthropogenically induced change. Reference sites are generally defined as those sites having minimal exposure to human activities and are representative of the waterbody type and region of interest (Hughes et al. 1986). Reference conditions, as used here, are numerical descriptions of the variability of biological measurements taken from a composite of multiple reference sites (Gibson et al. 1996).
Selection of relevant environmental descriptors. To obtain the most reliable and complete picture of the state of the environment, an integrated ecosystem-level monitoring approach should be adopted, involving coordinated chemical, physical, and biological sampling. The following physical and chemical environmental parameters are considered essential for the interpretation of macrozoobenthic data:
Organic carbon content in sediments. Gives an indication of food availability to benthic fauna. Can also provide an indication of the likely oxygen status of sediments.
Sediment granulometry (fractions expressed as % dry mass, median diameter, sorting coefficient). Indicates sediment homogeneity and sorting. Important in determining community composition and diversity and relationship to organic or contaminant content.
Dissolved oxygen in bottom water/sediments. Despite that momentary values are not indicative of the long-term oxygen conditions measurements during summer could provide detection and evidence of hypoxia/anoxia events. Oxygen concentration should be measured at the sediment-water interface or within the sediments since strong declining gradients of dissolved oxygen are usually observed within the benthic boundary layer (Diaz & Rosenberg, 1995).
Secchi disk transparency. Despite that momentary values are not indicative of the long-term conditions it is considered as the simplest and easiest to measure indicator of the trophic conditions.
Long-term chlorophyll-a concentration data. This is considered to be a good indicator of trophic status, but concentrations in coastal waters are subject to large influences by carry-over of phytoplankton from rivers and in the Black Sea, particularly, concentrations are highly depth-dependent.
Toxicants (selected heavy metals, petroleum hydrocarbons, chlorinated pesticides, etc.). These measurements and analyses are expensive, therefore should be done initially to establish the baseline concentrations and repeated at lower frequency compared to macrozoobenthos monitoring.
Depth. Determines the vertical gradients of dissolved oxygen and other gasses (i.e. H2S), trophic resources, light conditions, temperature, etc. and therefore governs the diversity and distribution of macrozoobenthic communities.
Temperature. Major physical variable important in determining the distribution of macrofauna.
Salinity. Major physical variable important in determining the diversity and distribution of macrofauna.
Adherence to standard protocols for sampling and analysis. Adoption of standard methodology for sampling and analysing macrozoobenthos on regional basis is of primary importance for data comparability and large-scale assessments.
4. Logistics
4.1Site location
Maps and charts to the appropriate scale should be obtained. The positions of sampling stations should be defined using geographic co-ordinates, e.g. latitude/longitude to at least two decimal points with reference to the appropriate system for graticules (such as European Datum: ED-50; World Geodetic Sytem: WGS-84). Latitude and longitude for sample sites should be determined prior to beginning field work (or should be the same as for sites surveyed in the pilot survey or previous monitoring studies). When revisiting sampling stations poorly-defined in terms of geographical coordinates, the water depth, known landmarks (unless in open sea) as well as sediment features should be used as the main criteria for relocating the sampling stations. A minimum accuracy of 50m and 20m in open waters and estuarine areas, respectively, should be attained.
4.2Sampling equipment
Remote sampling is most commonly carried out using a grab or corer. The nature of the sea bed will determine the most effective type of sampling gear. For soft bottom substrates, a grab of standard design is an appropriate sampler. Van Veen grab with a sampling area of 0.1m2 should be employed as a standard macrozoobenthos sampler for the Black Sea Integrated Monitoring and Assessment Programme (BSIMAP) since it is (i) an efficient sampler for the range of soft sediments encountered in the Black Sea, (ii) reliable and simple to operate and (iii) widely applied, which allows data comparison with other marine areas. Grabs should be equipped with hinged inspection ports. The biting depth of grabs can vary with sediment conditions. Weights can be added to adjust according to the sediment conditions.
4.3Vessel
The survey vessel should be appropriately and adequately equipped for bottom sampling, with sufficient deck space. The size of vessel required should be chosen as appropriate to the conditions in the sampling area and the type of sampling gear to be employed. In all cases where heavy sampling gear is deployed the vessel must be fitted with a suitable power winch, wire of the appropriate dimensions rigged to a meter wheel and an A frame or gantry. The vessel should also be equipped with echo-sounder or other appropriate depth measuring device and satellite global positioning device.
4.4Positioning equipment
A Differential Geographical Positioning System (DGPS) with monitor should be used in all sea areas if possible. If the differential is not available, accuracy should be assessed and a minimum of Global Positioning System (GPS) should be used.
4.5Personnel
van Veen Grab can be operated by two survey staff in addition to a winch operator. At least one of the survey team should be experienced with handling grabs and have experience of sampling and sieving marine invertebrates.
5. Ship-board routines
Sampling on shallow stations (70m or less) is recommended to be conducted during daytime, since some benthic species have semi-pelagic activity during the night.
5.1Grab deployment
At each site the grab should be set down gently at a speed that avoids triggering the mechanism. The winch wire should remaining vertical (wire angle must be kept as small as possible) to ensure an even bite of the grab. In the case of deep or fast-moving water this may require additional weights on the grab and maintaining position by motoring into the current or anchoring. Between approximately 5m and 10m above the sea floor, the lowering speed should be decreased (<0.5m/s) in order to further reduce the bow-wave and water turbulence in front of the grab. Contact with the sea floor is observed by the slack on the wire, after which the grab is gently raised for approximately the first 5m. Then the recovery can proceed at a faster speed.
Appropriate equipment for receiving and processing the samples should be ready on deck. On retrieval the grab should be placed on stable landing table. The sample should be examined for adequacy via the top inspection ports immediately upon retrieval on deck. If the sediment depth in the grab is less than 7cm in mud or 5cm in sand, the sample should be rejected. Other samples rejection criteria are given in the Recommendations for Quality Assurance, (see 8.4). If sediment characteristics make it impossible to collect approved samples, the best available samples should be retained, and the circumstances noted in the field record.
The faunal samples should be gently decanted into a receiving container (barrel).
The grab is to be rinsed thoroughly before redeployment.
Each laboratory shall regularly check the exact sampling area of its grab in order to make possible a correct calculation of the number of individuals per square metre.
Sediment characteristics and background information should be recorded before sieving.
5.2Sieving
Approved samples should be sieved in the field using seawater to remove fine sedimentary material. Each sample must be sieved, stored and documented separately.
The standard sieve for the Black Sea Integrated Monitoring and Assessment Programme (BSIMAP) shall be of metal gauze (stainless steel, brass or bronze) and have a mesh size of 1.0x1.0mm. In order to collect quantitatively developmental stages of the macrofauna and abundant smaller species (longer but thinner than 1mm) it is, however, recommended to use an additional sieve of mesh size 0.5x0.5mm. This additional sieve also ensures against lost of specimens when sieving because of using too high water pressure. The mesh size of the sieves has to be checked from time to time for damage and wear. The use of large sieves is encouraged because:
the risk of clogging is kept low;
the risk of spilling is reduced when transferring samples from the receiving container to the sieves.
Water should be added gently to the receiving container to produce a water sediment suspension. The use of sprinklers and hand-operated douches to suspend the sample is recommended. Very stiff clay can be gently fragmented by hand. The sample is transferred in small quantities to the sieve as a sediment-water suspension.
The sieving of the sample has to be done carefully in order to avoid damage of fragile animals. Sieving is done by washing the material in the sieve with gentle jets of seawater and shaking by hand. Deck hoses must be provided with shower nozzles. Visible fragile animals, e.g. some polychaetes, echinoderms, etc. or large, heavy molluscs shall be hand-picked during the sieving, placed in separate plastic bags/jars and fixed before being placed in the container along with the rest of the sample. Stones and big shells should be picked out and kept in separate containers or discarded if devoid of encrusting fauna to avoid the grinding effect.
In order to reduce damage of delicate organisms sieving may be done by placing the sieve in a water bath deep enough to cover the mesh screen and paddled until the sediments are washed out. However, this process is time consuming and therefore is not recommended in case of limited resources. Furthermore, long duration of sieving time should be avoided because small animals may actively pass through the sieve.
All residues retained on the sieve should be carefully flushed off the sieve with water from below into appropriate sample containers (e.g. plastic jars, plastic buckets with watertight lids).
Between the sample portions the sieves must be checked and cleared of trapped fauna and any sediment to avoid clogging and thus to ensure an equal mesh size during the whole sieving procedure.
According to the UKNMMP Green Book (2003) samples may be sieved to 0.5mm and 1mm fractions either in the field or in the laboratory and analysed separately. Whether separated in the field or laboratory, the sieving method employed should remain consistent from year to year.
Separation of 0.5mm and 1mm fractions in the laboratory is recommended because:
The time for sieving a sample onboard is kept shorter, which is important in time-limited cruises and in bad meteorological conditions (rain, cold, rough see).
Since the fractions must be kept separately, onboard separation will double the number of specimen containers, which is inconvenient for storage and transportation.
The risk of mixing up fractions from different samples increases if a sample is split in two specimen containers (for two fractions) onboard.
5.3Fixation
Samples (hand-picked animals and the sieving residue) should be fixed as soon as possible after sieving using buffered 37-41% formaldehyde (formalin). For small sample volumes, where no particularly large animals 4% formaldehyde:seawater solution should be appropriate. Where the sample contains debris, tube-dwelling polychaetes, large animals or a lot of residual sediment, especially in compact clay sediments, formalin concentration should be increased to 10% even 20 %.
There should be at least the same amount of solution in the sample container as solid material. Large shells may be opened to allow the fixative to penetrate to animal tissues. Fixative and sample material should be gently mixed by stirring or inverting the sample containers.
For buffering, two alternatives are recommended:
100g of hexamethylenetetramine (Hexamine = Urotropin) per 1dm3 of (40%) formaldehyde; or
1.5g sodiumtetraborate (=Borax) per 1 dm3 of (40%) formaldehyde.
Buffering is necessary to prevent the leaching of calcium from shell material within the sample.
All necessary measures should be taken to avoid health damage by formalin.
5.4Staining
Staining facilitates the sorting process and increases the sorting efficiency. However, over-staining may hinder identification of species. Staining is optional according to staff preference.
Rose Bengal (1 g/dm3 of 40% formaldehyde), which turns animal protein red is added to the fixation fluid. Alternatively the stain can be applied in the laboratory, where the sample should be washed free from formalin and then immersed in stain (1g Rose Bengal/dm3 of tap water + 5 g of phenol for adjustments to pH4-5) for 20 minutes.
5.5Labelling
The sample containers should be indelibly pre-marked with the unique sample information (station designation, sample number, replicate number and date) externally.
In addition samples should be properly labelled internally. The information filled in labels should be sufficient to identify the sample with certainty. The mandatory fields are date, station designation, depth, sample number, replicate number (additionally the cruise and vessel designations, type of grab, time, sediment type, etc. may be indicated). Labels made of heavy weight and chemically resistant paper should be filled in with a soft graphite pencil, which will not fade in Formalin. Filled in labels are placed inside the sample containers.
5.6Sample registering
Samples should be properly registered in sample recording sheets (the standard pro-forma for on-site records is given in Annex 1).
The following information should be recorded in the field:
project or contract identifier (code);
geographical coordinates for each sampling station;
type of positioning system and its accuracy;
whether or not a buoy was used;
whether or not the ship was anchored;
date and time of each sampling station/sample;
the water depth from which the sample was taken (if more than one sample is taken at a station, the depth range of samples should be recorded);
the name, type and sampling area of the sampler;
sieve mesh aperture sizes;
number of replicate samples;
depth of sediment in grab as a measure of sample volume;
comments such as rejected/unapproved samples together with the causes;
a visual sediment description, including:
- a description of sediment type (e.g. sand, silt, clay, etc. and their relative proportions), including important notes, e.g., main groups of large, easily visible species present, occurrence of concretions, stones, dead shells, etc.;
- surface colour and colour change down the sediment profile, if visible;
- smell, e.g. presence and severity of H2S;
- anthropogenic debris, rubbish, plastics.
near-bottom temperature and salinity;
person responsible for sampling.
6. Laboratory routines
6.1Sorting and taxonomic identification
Small portions of the unsorted material shall be put on a set of tightly connected sieves with mesh size 0.5mm and 1mm and washed with tap water under a fume extractor, so that sorters are not exposed to formalin vapour. When large megafauna (bivalves, gastropods, etc.) are present in the sample, use of a third sieve (mesh size 5mm) may be considered. Sample fractions should be analysed separately.
The sample material should always be sorted using suitable magnification (magnification lamp, stereo-microscope).
Initial sorting should be undertaken into four major taxonomic groups: segmented worms (Annelida), animals with shells (Mollusca), animals with jointed limbs (Arthropoda) and other marine invertebrates. These are placed in separate sample vials with identification labels. Once the major sorting has taken place, it is best that each group is identified to the lowest possible level by a specialised researcher. When identifying species there inevitably will be cases when specimens cannot be identified to species due to damage or unsolved taxonomic problems. In case of doubtful identification the lowest reliable taxonomic level should be given. If it is certain that only one species is found, then this is indicated by sp. following the genus (e.g. Capitella sp.), and if it is certain that more than one species is found then this is indicated by spp. (e.g. Capitella spp.).
The three major taxonomic groups Polychaeta, Mollusca and Crustacea should be identified to the species level. These are the richest groups in the Black Sea and generally contribute mostly to the abundance and biomass of macrozoobenthos.
Anthozoa, Echinodermata, Cephalochordata, Phoronidea and Pantopoda should also be identified to species level, since sufficient taxonomic expertise and keys are available in the Black Sea region.
Nemertini, Turbellaria, Oligochaeta, Chironomidae and insects in general may be identified to higher taxonomic level (Phylum or Class) for routine monitoring purposes.
Taxonomic guides and keys used for the identification of organisms should be reported with the data. The reference list of taxonomic literature to be employed for the identification of the Black Sea macrofauna is shown in Annex 3.
In order to overcome taxonomic discrepancies due to the use of synonyms common nomenclature shall be used according to the European Register of Marine Species (ERMS) available on http://www.marbef.org/data/ermssearch.php. This will facilitate comparison of data not only within the Black Sea region but also with other European seas.
A taxonomic reference collection should also be available for training and verification purposes.
A checklist of species encountered in the studied area should be established and regularly updated. A provisional list of species from the three major taxonomic groups Polychaeta, Crustacea and Mollusca is provided in Annex 4.
6.2Abundance determination
Broken animals shall only be counted as individuals by their heads (e.g. polychaetes) or hinges of bivalves with adhering pieces of tissue.
Taxa that are not sampled quantitatively or that are not truly indicative of sediment conditions shall not be quantified but their presence should be noted. These taxa include Foraminifera, Nematoda, planktonic organisms, benthic fish, and colonial epifauna (Poryfera, Bryozoa, etc.).
6.3Biomass determination
Biomass can be expressed in a variety of ways (e.g. wet weight, dry weight and ash-free dry weight). Wet weight analyses are preferable for routine and monitoring surveys. Analyses of dry weight and ash-free dry weight may be carried out in certain circumstances, but are not generally recommended for faunal studies as the material is destroyed in the process.
Recently collected material is kept in buffered fixative for a recommended period of three months before wet-weight analysis, to stabilise the mass. Practical issues relating to survey demands may dictate earlier analysis, in which case absolute values may be unreliable, but spatial information can still be informative. The wet weight is obtained by weighing after the external fluid has been removed on filter paper. The animals are placed on filter paper and moved around until no more wet traces are left behind, ensuring that undue pressure is not applied. Animals with shells are generally weighed with their shells; the water should be drained off bivalves before weighing. All tube dwelling species (polychaetes) should be removed from their tubes. Echinoids and ascidians should be punctured to drain off the water before blotting on filter paper. As soon as dry, the specimens are transferred to a tared container on the balance. Balance should be accurate to 0.0001g. After 30 seconds has elapsed the weight of animals is recorded to 0.0001 g. However, where a taxon weighs less than this, the weight is recorded as 0.0001g. The container should be re-tared before weighing the next taxon.
The dry weight shall be estimated after drying the formalin material at 60oC to constant weight (for 12-24 hours, or an even longer time, depending on the thickness of the material).
Ash-free dry weight should be estimated after measuring dry weight. It is determined after incineration at 500C in an oven until weight constancy is reached (~6 hours, depending on sample and object size). The temperature of the oven should be checked with a calibrated thermometer because there may be considerable temperature gradients (up to 50C) in a muffle furnace. Caution is advised to avoid exceeding a certain temperature (>550C), at which a sudden loss of weight may occur owing to the formation of CaO from the skeletal material of many invertebrates (CaCO3). This can reduce the weight of the mineral fraction by 44%. Before weighing, the samples must be kept in a desiccator while cooling down to room temperature after oven drying or removal from the muffle furnace.
7. Data reporting
Data obtained from laboratory analysis are entered into standard data report sheets (an example pro-forma is given in Annex 2). The following information should be included in scientific reporting:
complete list of taxa recorded, including those that are not quantified;
abundance (number of individuals) within each taxon;
biomass within each taxon;
appropriate metadata (e.g. location of sampling site, sampling depth, type and area of sampler, mesh size of sieves, etc.), which are necessary for the correct interpretation of data.
The final dataset obtained from a survey should be recorded as a taxon by abundance (or biomass) by station matrix in electronic spreadsheet format. Abundance and biomass data should be normalised per m2.
All data entered into electronic spreadsheets must be proof-read.
8. Recommendations for Quality Assurance
Quality assurance measures should focus on the following areas:
8.1Equipment calibration
The technical quality of the equipment should be verified on regular basis. The most important of these involve:
accuracy of depth and positioning fixing equipment;
sampling area of grab;
sieve aperture size;
recalibration of eyepiece graticules and microscope maintenance.
8.2Training
Experienced and well-trained personnel are a basic prerequisite for maintaining high level quality standards of sampling and analysing procedures. Proper training and education should be given to all staff involved in field and laboratory work, and documentation.
Ring tests and intercalibration exercises on a regional basis should be undertaken regularly and be obligatory for institutions delivering data to the Black Sea Integrated Monitoring and Assessment Programme (BSIMAP). They should be open to all institutions, including private industry. Technicians and taxonomic specialists who carry out the actual procedures rather than managing scientists should take part in the exercises.
8.3Repeatability of site positioning.
Exact positioning and correct depths when sampling are essential to note on the sample record sheet to avoid comparisons between samples taken at different localities (although noted as the same station in the protocols).
8.4Quality and quantity of the sample.
The criteria for sample rejection are as follows:
- less than 5 litres of sample volume is obtained by 0.1 m2 grab in soft sediments or less than 2.5 litres in hard-packed sand. (5l approximates to a depth of 7cm, while 2.5l approximates to a depth of 5cm. Measures of sample depth are taken vertically at the centre of the closed grab buckets.);
- incomplete closure is noted;
- an obvious uneven bite is noted;
- spillage during transferring of samples is observed;
- samples clearly deviate from the other samples (e.g., there is an observed change from clean sand samples to mussel bank samples). The samples should be nevertheless kept, in order to record faunal patchiness, but another sample should be taken to replace it in calculating the mean for the station.
Sieving of samples in water bath is recommended as the gentlest way of washing samples.
The use of large sieves is encouraged because:
- the risk of clogging is kept low;
- they reduce the risk of spilling when transferring samples from containers/buckets to the sieves.
8.5Accuracy and traceability of sample numbering and registration
Each sample must be sieved, stored and documented separately.
Samples should be properly marked externally and labelled internally to insure unambiguous identification.
Samples should be properly registered in standard sample recording sheets (an example pro-forma for on-site records is given in Annex 1).
8.6 Accuracy of sample sorting and taxon identification
It is advisable to stain the samples to facilitate sorting, if this does not hamper species identification.
The sorting efficiency of the personnel sorting the samples should be checked by an experienced sorter. At least 10% of the processed samples, randomly selected, should be subjected to quality control of the sorting efficiency. If laboratory staff are inexperienced, than the percentage should be increased accordingly.
The species determination should be checked by an experienced identifier. At least 10% of the processed samples, randomly selected, should be subjected to quality control of the identification efficiency. If the laboratory stuff is inexperienced, than the percentage should be increased accordingly.
A list of literature used for taxonomic identification should be compiled and reported with the data. The list should be updated regularly and should reflect recent advances in the taxonomic literature.
Regional taxonomic workshops and intercalibration exercises should be held on a regular basis and be attended by every laboratory.
A checklist of species in the area should be developed, distributed to the participating laboratories and updated regularly.
Specimens of each taxon should be placed in separate vials in reference collections. A separate reference collection may be required for individual surveys to make later taxonomic checks possible.
8.7Accuracy of data compilation
All datasets must be proof-read after input to the computer, before usage.
One way to check the quality of numbers in the database is to compare individual mean weights. If they are abnormally high or low, the figures should be verified.
8.8In-house Quality Assurance
It is recommended that organisations should prepare their own in-house procedures and training records, including, but not limited to, the following aspects of the work:
- records of training and experience of survey personnel;
- records of training and experience of laboratory staff;
- procedures for handling survey equipment;
- procedures for collection, processing and analysis of macrobenthic samples;
- procedures for recording biological and environmental data.
Signed protocols should be obligatory for all steps in the analyses.
Taxonomic certification of the persons responsible at the laboratories is recommended.
Independent re-analysis of samples of the benthic macrofauna by second researcher should be done for at least 10% of samples.
9.1Extraction efficiency total taxa target
To achieve a pass, the number of taxa extracted should be within 10% or 2 taxa (whichever is greater) of this total.
9.2 Extraction efficiency total individuals target
The total should be within 10% or 2 individuals (whichever is greater) of the total resulting from re-analysis of samples.
9.3Total wet weight biomass target
The total value should be 20% of the value obtained from re-analysis of the sample.
9.4Bray-Curtis comparison
Comparison of the two untransformed data sets, arising from the work of the participating laboratory and from independent re-analysis, should result in a Bray-Curtis Similarity Index of ≥90%.
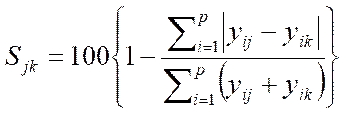 (IV.2.6)
(IV.2.6)
where Sjk Similarity index of samples j and k;
yij abundance of ith species in sample j;
yik ‑ abundance of ith species in sample k;
Data flags are applied using a graded system related to the untransformed Bray-Curtis scores as follows:
100% BCSI: Excellent
95-<100% BCSI: Good
90-95% BCSI: Acceptable
85-90% BCSI: Poor remedial actions suggested
<85% BCSI: Fail - remedial actions required
If the results obtained from the re-analysis of samples do not meet the set targets, than all the samples in the batch should be re-quantified after remedial actions have been taken.
10. Data analysis and metrics
Metrics are primary variables or derivative indices that describe community attributes (biological descriptors) in terms of species richness/composition, quantity (abundance/biomass), structure and function.
The approaches that have been developed in order to explain and reveal the impact of pressures (physical, chemical and biological) on benthic communities can be grouped into three classes:
univariate measures/indices such as number of species, abundance, biomass; diversity indices based on richness/abundance counts; taxonomic diversity indices based on the taxonomic spread of species; biotic indices based on functional attributes such as trophic mode or ecological strategy; abundance/biomass ratios, etc.;
multi-metric indices combining several measures of community response to stress into a single index;
multivariate methods describing the assemblages pattern, including modelling.
Provisional list of macrozoobenthos metrics/indices to be tested in the environmental status assessment of the Black Sea is given below:
Primary measures:
- Number of taxa
- Presence/absence of identified sensitive species
- Abundance (total, of identified sensitive/tolerant/opportunistic taxa)
- Biomass (total, of identified sensitive/tolerant/opportunistic taxa)
Ratios and proportions:
- Total abundance/total biomass ratio
- Proportion of sensitive/tolerant/opportunistic taxa from the total number of taxa, abundance or biomass
Diversity indices:
- Margalefs Species richness index, d (Margalef, 1958)
- Pielous evenness index, j (Pielou, 1966)
- Simpson's dominance index, c (Simpson, 1949)
- Shannon-Wiener diversity index H (Lloyd, Zar and Karr, 1968)
- Expected number of species (ES) (Hurlbert, 1971)
- Average taxonomic diversity D (Warwick & Clarke 1995, 1998)
- Average taxonomic distinctness D+ (Warwick & Clarke 1995, 1998, Clarke & Warwick 1998)
- Variation in taxonomic distinctness L+ (Warwick & Clarke 2001, Clarke & Warwick 2001)
Biotic indices:
- Infaunal Trophic Index (ITI) (Word, 1978)
- Biological Quality Index (BQI) (Wilson et al., 1985)
- AZTI Marine Biotic Index (AMBI) (Borja et al., 2000)
- BENTIX (Simboura, Zenetos, 2002)
- Benthic Quality Index (BQI)(Rosenberg et al., 2004)
Multi-metric indices:
- Benthic Index of Biotic Integrity (B-IBI) (Weisberg et al., 1997).
Multivarite approaches. A range of data analyses procedures is available, extensively described in Clarke and Warwick (1994). Hierarchical clustering and ordination analyses have been recommended for assessments of benthic faunal data sets. Such techniques are most effective in the analysis of large data sets based on many samples and allow direct linkages to be made between biological variance and changes in specific environmental factors, including the ranking of such factors in order of importance.
Important remarks concerning the implementation of the above metrics/indices and multivariate statistical analysis methods include:
Preliminary adaptation of the biotic and multimetric indices (ITI, AMBI, BENTIX and B-IBI) is necessary for their implementation in the Black Sea. This involves allocation of Black Sea species to respective trophic/ecological groups, selection and statistical testing of candidate metrics to be included in B-IBI.
Reference values of community metrics (reference conditions) at different seabed habitats should be established.
Threshold values of community metrics that delineate acceptable from unacceptable ecological status should be defined.
Comprehensive list of Black sea species shall be compiled for the calculation of the taxonomic indices (D, D+, L+).
Relevant statistical packages include PRIMER, AMBI, Bio Diversity Programme.
11. Acknowledgements
The preparation of this guideline was supported by GEF-UNDP Project PIMS 3065: Control of eutrophication, hazardous substances and related measures for rehabilitating the Black Sea ecosystem: Phase 2 (BSERP).
We wish to gratefully acknowledge the contribution of the following persons for the compilation of the Provisional check list of macrozoobenthic Polychaeta, Crustacea and Mollusca encountered in the Black Sea and Azov Sea (Annex 4): Camelia Dumitrache1 (Mollusca), Christos Arvanitidis2 (Polychaeta), Dragos Micu3 (Mollusca), Nikolai Revkov4 (Polychaeta), S. nsal Karhan5 (Crustacea, Mollusca), Valentina Todorova6 (Polychaeta), Victor Surugiu7 (Polychaeta).
1 National Institute for Marine Research and Development Grigore Antipa
300 Mamaia Blv.
Ro-900581, Constanta, 3
Romania
2 Institute of Marine Biology and Genetics,
Hellenic Center for Marine Research,
Former American Base of Gournes,
71003, Heraklion, Crete,
Greece.
3 National Institute for Marine Research and Development Grigore Antipa
300 Mamaia Blv.
Ro-900581, Constanta, 3
Romania
4 Institute of Biology of Southern Seas, National
Academy of Science
2, Nakhimov Av., 99011 Sevastopol
Crimea, Ukraine
revkov@ibss.iuf.net
5 Istanbul University Institute of Marine Science and Management
Muskule Sok., №: 1
34116, Vefa-Istanbul
Turkey
6 Institute of Oceanology-BAS
9000 Varna
P.O.BOX 152
Parvi maj Str., № 40
Bulgaria
7Al. I. Cuza University of Iaşi, Faculty of Biology
Bd. Carol I, 20A,
6600, Iaşi
Romania
Cover photograph was generously provided by Lyubomir Klissurov:
Institute of Oceanology-BAS
9000 Varna
P.O.BOX 152
Parvi maj Str., № 40
Bulgaria
www.klissurov.dir.bg
12. References
Borja, A., Franco, J., Prez, V., 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within european estuarine and coastal environments. Mar. Pollut. Bull. 40 (12), 11001114.
Clark, K.R. and Ainsworth, M., 1993. A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series, 92, 205-219.
Clarke, K. R. and Warwick, R. M., 1994. Changes in marine communities: an approach to statistical analysis and interpretation. Natural Environmental Research Council, Plymouth.
Clarke, K.R. & Warwick, R.M., 1998. A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology, 35, 523-531.
Clarke, K.R. & Warwick, R.M., 2001. A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Marine Ecology - Progress Series, 216, 265-278.
Davies J., Baxter J., Bradley M., Connor D., Khan J., Murray E., Sanderson W., Turnbull C. and Vincent M., 2001. Marine Monitoring Handbook, ISBN 1 86107 5243, 405 pp.
Eleftheriou, A. and Holme, N.A., 1984. Macrofauna techniques. p. 140-216. In: Methods for the study of marine benthos (N.A. Holm and A.D. McIntyre, eds.). Blackwell Scientific Publications, Oxford, 387 pp.
Gibson, G. R., M. T. Barbour, J. B. Stribling, J. Gerritsen, and J. R. Karr. 1996. Biological criteria: technical guidance for streams and small rivers. EPA 822-B-96-001. U.S. Environmental Protection Agency, Office of Water, Washington, DC.
Gray, J. S., McIntyre, A.D., and tirn, J., 1992. Manual of methods in aquatic environment research. Part 11. Biological assessment of marine pollution with particular reference to benthos. FAO Fisheries Technical Paper 324: 49 pp.
Holme, N.A. & McIntyre, A., 1984. Methods for the Study of Marine Benthos. Oxford, 387 pp.
Hughes, R. M., D. P. Larsen and J. M. Omernik. 1986. Regional reference sites: a method for assessing stream pollution. Environmental Management 10: 629-635.
Hurlbert, S.H., 1971. The nonconcept of species diversity: A critique and alternative parameters. Ecology 52: 577-586.
ICES. 2004. Report of the ICES/OSPAR Steering Group on Quality Assurance of Biological Measurements in the Baltic Sea. 59 pp.
ICES. 2005. Report of the ICES/OSPAR Steering Group on Quality Assurance of Biological Measurements in the Northeast Atlantic. 59pp.
ISO 16665: 2005 (E). Water quality Guidelines for quantitative sampling and sample processing of marine soft-bottom macrofauna.
Jeffrey. D. W., Wilson, J. G., Harris, C. R. and D.L. Tomlinson, 1985. The application of two simple indices to Irish estuary pollution status. Estuarine management and quality assessment. Plenum Press, London. 147-165 pp.
Kucheruk N., 2004. Manual macro Zoobenthos. http://bsc-onlinedocs.ath.cx/Zoobenthos-EN/
Lloyd, H., Zar, J.H., and Karr, J.R., 1968. On the calculation of information - theoretical measures of diversity. Am. Mid Nat. 79, 257-272.
Manual for Marine Monitoring in the COMBINE Programme of HELCOM, 2003. Part C. Programme for monitoring of eutrophication and its effects. Annex C-8 Soft bottom macrozoobenthos. http://sea.helcom.fi/Monas/CombineManual2/PartC/CFrame.htm
Margalef R., 1958. Information theory in ecology, Gen. Syst., 3, 36-71.
McIntyre, A. D., 1978. The Benthos of the western North Sea. Rapp. P.-v. Run. Cons. Int. Explor. Mer, 172: 405-417.
Pielou, E.C., 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol., 13, 131-144.
Rees, H.L., 2004. Biological monitoring: General guidelines for quality assurance. ICES Techniques in Marine Environmental Sciences, No. 32. 44 pp.
Rees, H.L., C. Heip, M. Vincx and Parker M.M., 1991. Benthic communities: use in monitoring point-source discharges. ICES Techniques in Marine Environmental Sciences No. 16, 70pp.
Rosenberg, R., M. Blomqvist, H. Nilsson, H. Cederwall and A. Dimming, 2004. Marine quality assessment by use of benthic species-abundance distribution; a proposed new protocol within the EC Water Framework Directive. Marine Pollution Bulletin.
Rumohr, H., 1990. Soft bottom macrofauna: collection and treatment of samples. ICES Techniques in Marine Environmental Sciences No. 8, 18pp.
Rumohr, H., 1999. Soft bottom macrofauna: Collection, treatment, and quality assurance of samples. ICES Techniques in Marine Environmental Sciences No. 27, 26 pp.
Simboura N., Zenetos A., 2002. Benthic indicators to use in Ecological Quality classification of Mediterranean soft bottom marine ecosystems, including a new Biotic Index. Mediterranean Marine Science, 3/2, 77-111.
Simpson, E.H., 1949. Measurement of diversity. Nature, Lond. 163, 688.
UK National Marine Monitoring Programme Green Book, 2003. http://www.sepa.org.uk/marine/index.htm
Warwick, R.M. and Clarke, K.R., 1995. New "biodiversity" measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology Progress Series, 129: 301-305.
Warwick, R.M. & Clarke, K.R., 1998. Taxonomic distinctness and environmental assessment. Journal of Applied Ecology, 35, 532-543.
Warwick, R.M. & Clarke, K.R., 2001. Practical measures of marine biodiversity based on relatedness of species. Oceanography and Marine Biology: an Annual Review, 39, 207-231.
Weisberg, S. B., Ranasinghe, J. A., Dauer, D. M., Schaffner, L. C., Diaz, R. J., and Frithsen., J. B., 1997. An estuarine benthic index of biotic integrity (B-IBI) for the Chesapeake Bay. Estuaries 20:149-158.
Word, J. Q., 1979. The Infaunal Trophic Index. Sth Calif. Coast. Wat. Res. Proj. Annu. Rep., El Segundo, California. 19-39.
SAMPLE RECORD - MACROZOOBENTHOS
No
|
Project/contract identifier. Cruise duration: from .. to . Vessel (name and type1): . Positioning system2: Sampling precision3: Sediment sampler type/area: . Mesh size of sieves (mm): . Date: . Time: Station/site designation: Sample №: . Replicate №: Coordinates: Lat.: Long.: Depth (m):. Depth of sediment in sampler (cm): Т Н20 bottom (оС) S H20 bottom (о/оо). Sediment type4 and observations5: . . . . Person responsible for collecting: .
|
Annex 2
DATA REPORT SHEET - MACROZOOBENTHOS
Project/contract identifier: .
Vessel (name and type1): .
Positioning system2: .
Sampling precision3: .
Date: .
Time:
Station/site designation: .
Coordinates:
Depth (m): .
Sample №:
Replicate №: .
Sediment sampler type/area:
Mesh size of sieves (mm):
Sediment type4 and observations5: ..
Т Н20 bottom (оС):
S H20 bottom (о/оо): .
Person responsible for collecting: .
|
№ |
Taxon |
Abundance (number of individuals in sample) |
Biomass (grams in sample) |
|
1 |
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
|
5 |
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
n |
|
|
|
|
|
Total: |
|
|
List of taxonomic literature used:.
Sorter:
Identifier: .
Date: .
Notes:
1 Vessel type: C = commercial, R = research, O = other
2 Positioning system: DEC = DECCA, DGP = Differential Global Positioning System, GPS = Global positioning System
3 Sampling precision: A = ship anchored, B = buoy used, N = nether of the above used
4 Sediment type: Mud, Sandy mud, Muddy sand, Sand, Gravelly sand, Sandy gravel, Gravel; Fluid, Soft, Firm
5 Observations: colour, smell, surface relief, depth of RPD layer, accretions (e.g. coal, illmenite, etc.), dominant fauna, presence of burrows, tubes, etc.
Annex 3
List of taxonomic literature to be employed for the identification of macrozoobenthic species in the Black Sea
Bacescu, M., 1951. Cumacea. In: Fauna Romaniei, 1(1), Ed. Academiei Romane, 1-94, Bucuresti.
Bacescu, M., 1982. Contributions la connaissance des Cumacs de la Mer de Marmara et dEge (Ile Eubea). Travaux Museum dHistoire naturelle Grigore Antipa, 24, 45-54.
Ball, I.R. & Reynoldson, T.B., 1981. British planarians. Synopses Br. Fauna (N.S.) No. 19. Linnean Society of London. Estuarine and Brackish Water Sciences Association. Bath: Cambridge University Press.
Bellan-Santını, D., Dıvıacco, G., Krapp-schıckel, G., Myers, A.A. and Ruffo, S., 1989. Part 2. Gammaridea (Haustoriidae to Lysianassidae [Ruffo, S. (Ed.) The Amphipoda of the Mediterranean, Mmoires de lInstitut ocanographique, 13, 365-576, Monaco]
Bellan-Santını, D., Karaman, G., Krapp-Schıckel, G., Ledoyer, M. and Ruffo, S., 1993. Part 3. Gammaridea (Melphidippidae to Talitridae), Ingolfiellidea, Caprellidea. [Ruffo, S. (Ed.) The Amphipoda of the Mediterranean, Mmoires de lInstitut ocanographique, 13, 577-813, Monaco]
Bellan-Santını, D., Karaman, G., Krapp-Schıckel, G., Ledoyer, M., Myers, A.A., Ruffo, S. and Schıecke, U., 1982. Part 1. Gammaridea (Acanthonotozomatidae to Gammaridae) [Ruffo, S. (Ed.) The Amphipoda of the Mediterranean, Mmoires de lInstitut ocanographique, 13, 1-364, Monaco]
Bellan-Santını, D., Karaman, G., Ledoyer, M., Myers, A.A., Ruffo, S. and Vader, W., 1998. Localities and Map, Addenta to Parts 1-3, Ecology, Faunistics and Zoogeography, Bibliography, Index. [Ruffo, S. (Ed.) The Amphipoda of the Mediterranean, Mmoires de lInstitut ocanographique, 13, 814-959, Monaco]
Bouvıer, E.L., 1923. Pycnogonides. Faune de France, Vol. 7, Paris.
Day, J.H., 1967. A monograph on the Polychaeta of southern Africa. Trustees of British Museum (Natural History), London.
Diaz, R. J. & Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
Ergen, Z., ınar, M.E. and Ergen, G., 2000. On the Nereididae (Polychaeta: Annelida) of the Bay of Izmir. Zoology in the Middle East, 21, 139-158.
Fage, L., 1951. Cumacs. Faune de France, Vol. 54, Paris.
Fauchald, K., 1977. The polychaet worms. Definitions and keys to the orders, families and genera. Natural History Museum, Los Angles Country, Sci. Ser. 28.
Fauvel, P., 1923. Polychtes errantes. Faune de France, Vol. 5, Paris.
Fauvel, P., 1927. Polychtes sdenteires. Faune de France, Vol. 16, Paris.
Gaıllard, J.M., 1987. Gasteropodes. [Fischer, W., Schneider, M. ve Bouchot, M.L. (Ed.) Fishes FAO didentification des especes pour les besoins de la peche. Mditerrane et Mer Noire, Zone de peche 37, Vol. I.: Vgetaux et invertebrs, 312-367, Rome]
Ghisotti, F., Melone, G., 1972. Cotalogo Illustrate delle Conchiglie Marine del Mediterraneo 4. Trochidae, Conchighie VIII., 1112., 4777.
Ghisotti, F.Melone, G., 1971. Catalogo Illustrato delle Conchiglie Marine del Mediterraneo 3. Trochidae, Conchighie VII., 12., 4777.
Ghisotti, F.Melone, G., 1975. Catolago Illustrate delle Condhiglie Marine del Mediterraneo,5 Trochidae, Conchighie IX., 1112., 147208.
Hayward, P.J. and Ryland, J.S. (Ed.), 1996. Handbook of the marine fauna of North-West Europe. Oxford University Press.
Holthuıs, L.B., 1987. Vrais Crabes. [Fischer, W., Schneider, M. and Bouchot, M.L. (Ed.) Fishes FAO didentification des especes pour les besoins de la peche. Mditerrane et Mer Noire, Zone de peche 37, Vol. I.: Vgetaux et invertebrs, 312-367, Rome]
21. Kırkım, F., 1998. Ege Denizi Isopoda (Crustacea) faunasının sistematiği ve ekolojisi zerine araştırmalar. Doktora Tezi, Ege niversitesi., İzmir.
Koehler, R., 1921. Echinodermes. Faune de France, Vol. 1, Paris.
Manuel, R.L., 1987. British Anthozoa (Coelenterata: Octocoralia and Hexacoralia). Synopses of the British Fauna, new ser., no:18.
Nordsıeck, F., 1972. Die europischen Meereschnecken (Opisthobranchia mit Pyramidellidae, Rissoacae) von Eismeer bis Kapverden, Mittelmeer und Schwarzes Meer. Gustav Fischer Verlag, Stuttgart.
Parenzan, P., 1970. Carta didentit delle conchiglie del Mediterraneo,Vol. I, Gasteropodi, Ed. Bios Taras, Taranto.
Parenzan, P., 1974. Carta didentit delle conchiglie del Mediterraneo, Vol. II, Bivalvi, Prima Parte, Ed. Bios Taras, Taranto
Parenzan, P., 1976. Carta didentit delle conchiglie del Mediterraneo, Vol. II, Bivalvi, Seconda Parte, Ed. Bios Taras, Taranto.
Poppe, G.T. ve Goto, Y., 1993. European seashells. Vol. 2 (Scaphopoda, Bivalvia, Cephalopoda). Verlag Christa Hemmen, Wiesbaden.
Poutıers, J.M., 1987. Bivalves. [Fischer, W., Schneider, M. ve Bouchot, M.L. (Ed.) Fishes FAO didentification des especes pour les besoins de la peche. Mditerrane et Mer Noire, Zone de peche 37, Vol. I.: Vgetaux et invertebrs, 312-367, Rome]
Preda, S., 1995. Diversitatea Lumii VII. Determinatorul Ilustrat al florei Si faunei Romaniei. Vol. I Medul Marin.
Rıedl, R., 1983. Fauna und Flora des Mittelmeeres. Verlag Paul Parey, Hamburg und Berlin.
Tebble, N., 1966. British bivalve seashells. Trustees of British Museum (Natural History) London.
Tortonese, E., 1965. Echinodermata. Fauna dItalia. Vol. 6. Edizioni Calderini, Bologna.
Walker-Smıth, G.K. and Poore, G.C.B., 2001. A phylogeny of the Leptostraca (Crustacea) with keys to families and genera. Memoirs of Museum Victoria, 58 (2), 383-410.
Zarıquıey Alvarez, R., 1968. Crustceos Decpodos Ibricos. Invesigacin Pesquera, no: 32, Barcelona.
Гурьянова Е.Ф., 1951. Бокоплавы морей СССР. Определители по фауне СССР, вып.41. Изд-во. АН СССР.
Жадин В. И., 1952. Моллюски пресных и солоноватых вод СССР. Определители по фауне СССР, вып. 46. Изд-во АН СССР.
Киселева М.И., 2004. Многощетинковые черви (Polychaeta) Черного и Азовского морей. Изд-во Кольского научного центра РАН, 409 с.
Маринов Т., 1977. Фауна на България т. 6, Многочетинести червеи (Polychaeta), Издателство на БАН, София, 258 стр.
Мордухай-Болтовской Ф. Д., 1968. Определитель фауны Черного и Азовского морей, Том первый, Наукова думка, Киев, 437 с.
Мордухай-Болтовской Ф. Д., 1969. Определитель фауны Черного и Азовского морей, Том второй, Наукова думка, Киев, 536 с.
Мордухай-Болтовской, Ф. Д., 1972. Определитель фауны Черного и Азовского морей, Том третий, Наукова думка, Киев, 340 с.
Annex 4
Provisional check list of macrozoobenthic Polychaeta, Crustacea and Mollusca encountered in the Black Sea and Azov Sea
POLYCHAETA
Chloeia venusta Quatrefages, 1865
Arenicola marina (Linnaeus, 1758)
Arenicolides branchialis (A. & M. Edwards, 1833)
Capitella capitata (Fabricius, 1780) capitata Warren, 1976
Capitella giardi (Mesnil, 1897)
Capitella minima Langerhans, 1880
Capitellethus dispar (Ehlers, 1907)
Dasybranchus caducus (Grube, 1846)*
Dasybranchus gajolae Eisig, 1887*
Heteromastus filiformis (Claparede, 1864)
Notomastus latericeus M. Sars, 1851
Notomastus lineatus Claparede, 1870
Notomastus profundus Eisig, 1887
Clymenura clypeata (Saint-Joseph, 1894)
Euclymene collaris (Claparede, 1870)
Euclymene oerstedi (Claparede, 1863)
Euclymene palermitana (Grube, 1840)
Macroclymene santadarensis (Rioja, 1917)
Maldane glebifex Grube, 1860
Micromaldane ornithochaeta Mesnil, 1897
Petaloproctus terricola Quatrefages, 1865
Praxillella praetermissa (Malmgren, 1866)*
Ctenodrilus serratus (Scmidt, 1857)
Stygocapitella subterranea Knollner, 1934
Dinophilus gyrociliatus O. Schmidt, 1857
Trilobodrilus heideri Remane, 1925
Drilonereis filum (Claparede, 1868)
Dorvillea rubrovittata (Grube, 1855)
Protodorvillea kefersteini (McIntosh, 1869)
Schistomeringos neglecta (Fauvel, 1923)
Schistomeringos rudolphi (Chiaje, 1828)
Eunice harassii A. & M. Edwards, 1833*
Eunice vittata (Chiaje, 1829)
Lysidice ninetta A. & M. Edwards, 1833
Marphysa belli (A. & M. Edwards, 1833)*
Nematonereis unicornis (Grube, 1840)
Lumbrineris gracilis (Ehlers, 1868)*
Lumbrineris latreilli A. & M. Edwards, 1834*
Scoletoma tetraura (Schmarda, 1861)*
Aponuphis bilineata (Baird, 1870)*
Nothria conchylega (M. Sars, 1835)*
Onuphis eremita A. & M. Edwards, 1833*
Nerilla antennata O. Schmidt, 1848
Armandia cirrhosa Philippi, 1861*
Ophelia bicornis Savigny, 1818
Ophelia limacina (Rathke, 1843)
Polyophthalmus pictus (Dujardin, 1839)
Naineris laevigata (Grube, 1855)
Orbinia cuvierii (A. & M. Edwards, 1833)
Orbinia latreillii (A. & M. Edwards, 1833)
Protoaricia oerstedii (Claparede, 1864)
Aricidea claudiae Laubier, 1967
Levinsenia gracilis (Tauber, 1879)
Paradoneis lyra (Southern, 1914)
Paraonides neapolitana Cerruti, 1909
Cirrophorus lyriformis (Annenkova, 1934)*
Paraonis fulgens (Levinsen, 1884)
Myriochele heeri Malmgren, 1867
Owenia fusiformis Chiaje, 1842
Vigtoriella zaikai Kisseleva, 1992
Glycera alba (O. F. Muller, 1776)
Glycera capitata Oersted, 1843
Glycera gigantea Quatrefages, 1865
Glycera rouxi A. & M. Edwards, 1833
Glycera tesselata Grube, 1863
Glycera tridactyla Schmarda, 1861
Glycera unicornis Savigny, 1818
Goniadella bobretzkii Annenkova, 1929
Hesionides arenaria Friedrich, 1937
Kefersteinia cirrata (Keferstein, 1862)*
Microphthalmus fragilis Bobretzky, 1870
Microphthalmus sczelkowii Mecznikow, 1865
Microphthalmus similis Bobretzky, 1870
Micronephtys stammeri (Augener, 1932)
Nephtys caeca (Fabricius, 1780)
Nephtys ciliata (O. F. Muller, 1776)
Nephtys cirrosa Ehlers, 1868
Nephtys hombergii Savigny, 1818
Nephtys hystricis McIntosh, 1900
Nephtys incisa Malmgren, 1865*
Nephtys longosetosa Oersted, 1842
Nephtys paradoxa Malm, 1874
Ceratonereis costae (Grube, 1840)
Eunereis longissima (Johnston, 1840)
Nereis (Hediste) diversicolor (O. F. Muller, 1776)
Namanereis littoralis (Grube, 1872)
Namanereis pontica (Bobretzky, 1872)
Neanthes fucata (Savigny, 1818)
Neanthes succinea (Frey & Leuckart, 1847)
Nereis pelagica Linnaeus, 1758
Nereis rava Ehlers, 1868
Nereis zonata Malmgren, 1867
Perinereis cultrifera (Grube, 1840)
Platynereis coccinea (Chiaje, 1841)*
Platynereis dumerilii (A. & M. Edwards, 1833)
Websterinereis glauca (Claparede, 1870)
Pholoe inornata Johnston, 1839
Pholoe synophthalmica Claparde, 1868
Eteone picta Quatrefages, 1865
Eteone syphonodonta (Chiaje, 1822)
Eulalia viridis (Linnaeus, 1767)
Eulalia clavigera (A. & M. Edwards, 1834)
Eumida sanguinea Oersted, 1843
Hesionura coineaui (Laubier, 1962)
Nereiphylla nana Saint-Joseph, 1906
Nereiphylla paretti Blainville, 1828
Nereiphylla rubiginosa (Saint-Joseph, 1888)
Phyllodoce laminosa Savigny, 1818
Phyllodoce (Anaitides) lineata (Claparede, 1870)
Phyllodoce (Anaitides) maculata (Linnaeus, 1767)
Phyllodoce madeirensis Langerhans, 1880*
Phyllodoce (Anaitides) mucosa Oersted, 1843
Phyllodoce vittata Ehlers, 1864
Phyllotethys koswigi La Greca, 1949
Pseudomystides limbata Saint-Joseph, 1888
Pterocirrus limbata Claparede, 1888
Pterocirrus macroceros (Grube, 1860)
Sigambra tentaculata (Treadwell, 1941)
Pisione remota (Southern, 1914)
Acholoe astericola (Chiaje, 1828)*
Harmothoe extenuata (Grube, 1840)
Harmothoe gilchristi Day, 1960
Harmothoe imbricata (Linnaeus, 1767)
Harmothoe reticulata (Claparede, 1870)
Harmothoe spinifera (Ehlers, 1864)*
Lepidasthenia maculata Potts, 1910*
Lepidonotus carinulatus (Grube, 1870)*
Lepidonotus squamatus (Linnaeus, 1758) *
Polynoe scolopendrina Savigny, 1818
Subadyte pellucida (Ehlers, 1864)*
Labioleanira yhleni (Malmgren, 1867)*
Sthenelais boa (Johnston, 1839)
Ephesiella peripatus (Claparede, 1863)
Sphaerodoridium claparedeii (Greeff, 1866)
Sphaerodorum gracilis (Rathke, 1843)
Amblyosyllis formosa (Claparede, 1863)
Autolytus rubrovittatus Claparede, 1864
Brania tenuicirrata (Claparede, 1864)
Ehlersia cornuta (Rathke, 1843)
Exogone hebes (Webster & Benedict, 1884)
Exogone naidina Oersted, 1845
Grubeosyllis clavata (Claparede, 1863)
Grubeosyllis limbata (Claparede, 1868)
Haplosyllis spongicola (Grube, 1855)
Myrianida prolifer (O.F. Muller, 1788)
Myrianida brachycephala (Marenzeller, 1874)
Myrianida edwarsi Saint-Joseph, 1888
Petitia amphophthalma Siewing, 1955
Pionosyllis lamelligera Saint-Joseph, 1886
Pionosyllis pulligera (Krohn, 1852)
Proceraea aurantiaca Claparede, 1868
Proceraea cf. picta Ehlers, 1864
Pseudosyllis brevipennis Grube, 1863*
Sphaerosyllis bulbosa Southern, 1914
Sphaerosyllis claparedei Ehlers, 1864*
Sphaerosyllis hystrix Claparede, 1863
Sphaerosyllis ovigera Langerhans, 1879*
Streptosyllis varians Webster & Benedict, 1887
Syllides fulvus (Marion & Bobretzky, 1875)
Syllides longocirrata Oersted, 1845
Syllis amica Quatrefages, 1865*
Syllis armillaris (O. F. Muller, 1776)*
Syllis cf. compacta Gravier, 1900
Syllis gerlachi Hartmann-Schroeder, 1960
Syllis gracilis Grube, 1840
Syllis hyalina Grube, 1863
Syllis krohni Ehlers, 1864
Syllis monilaris Savigny, 1818
Syllis prolifera Krohn, 1852
Syllis variegata Grube, 1860
Syllis vittata (Grube, 1840)*
Xenosyllides violacea Perejaslavzeva, 1891
Trypanosyllis zebra (Grube, 1840)
Polygordius neapolitanus Frainpont, 1884
Protodrilus flavocapitatus (Uljanin, 1877)
Protodrilus purpureus (Schneider, 1868)
Saccocirrus papillocercus Bobretzky, 1872
Sabellaria taurica (Rathke, 1837)
Chone collaris Langerhans, 1880*
Chone filicaudata Southern, 1914*
Fabricia stellaris caspica (Zenkevitch, 1922)
Fabricia stellaris adriatica (Banse, 1956)
Jasmineira caudata Langerhans, 1880
Manayunkia caspica Annenkova, 1929
Oriopsis armandi (Claparede, 1864)
Potamilla torelli Malmgren, 1866
Ditrupa arietina (O. F. Muller, 1776)
Ficopomatus enigmaticus (Fauvel, 1923)
Hydroides norvegicus Gunnerus, 1768
Pomatoceros triqueter (Linnaeus, 1767)
Serpula vermicularis Linnaeus, 1767
Vermiliopsis straticeps (Grube, 1862)
Salmacina incrustans Claparede, 1868
Janua (Dexiospira) pagenstecheri (Quatrefages, 1865)
Neodexiospira pseudocorrugata (Bush, 1904)
Pileolaria militaris (Claparede, 1868)
Aphelochaeta marioni (Saint-Joseph, 1894)
Caulleriella bioculata (Keferstein, 1862)
Caulleriella caputesocis (Saint-Joseph, 1894)
Cirratulus cirratus (O. F. Muller, 1776)*
Cirriformia tentaculata (Montagu, 1808)
Protocirrineris chrysoderma Claparede, 1868
Timarete anchylochaeta (Schmarda, 1861)*
Timarete filigera (Chiaje, 1828)
Magelona mirabilis (Johnston, 1845)
Magelona papillicornis Muller, 1858 (sensu Fauvel, 1927)
Magelona rosea Moore, 1907
Aonides oxycephala (M. Sars, 1862)
Aonides pauchibranchiata Southern, 1914
Laonice cirrata (M. Sars, 1851)
Malacoceros fuliginosus (Claparede, 1868)
Malacoceros vulgaris (Johnston, 1827)
Malacoceros tetraceros (Schmarda, 1861)
Microspio mecznikowianus (Claparede, 1868)
Parascolelepis tridentata (Southern, 1914)
Polydora caulleryi Mesnil, 1897
Polydora ciliata (Johnston, 1838)
Polydora cornuta Bosc, 1802
Polydora websteri Hartman, 1943
Prionospio cirrifera Wiren, 1883
Prionospio malmgreni Claparede, 1870
Prionospio multibranchiata Berkeley, 1927
Pseudopolydora antennata (Claparede, 1868)
Pygospio elegans Claparede, 1863
Scolelepis cantabra (Rioja, 1918)
Scolelepis cirratulus (Delle Chiaje, 1828)
Spio decoratus Bobretzky, 1870
Spio filicornis (O. F. Muller, 1776)
Spio multioculata (Rioja, 1918)
Spiophanes bombyx (Claparede, 1870)*
Streblospio shrubsolii (Buchanan, 1890)
Sternaspis scutata (Renier, 1807)
Amage adspersa (Grube, 1863)
Amphicteis gunneri (M. Sars, 1835)
Hypania invalida (Grube, 1860)
Hypaniola kowalewskii (Grimm in Grube, 1887)
Melinna palmata Grube, 1870
Pectinaria belgica (Pallas, 1766)
Pectinaria koreni (Malmgren, 1866)
Pectinaria neapolitana Claparede, 1870
Petta pusilla Malmgren, 1866*
Amphitritides gracilis (Grube, 1860)
Nicolea venustula (Montagu, 1818)*
Polycirrus aurantiacus Grube, 1860*
Polycirrus caliendrum Claparede, 1868
Polycirrus haematodes (Claparede, 1864)
Polycirrus jubatus Bobretzky in Annenkova, 1924
Polycirrus pallidus (Claparede, 1864)
Proclea grafii (Langerhans, 1884)
Streblosoma bairdi (Malmgren, 1865)*
Thelepus triserialis (Grube, 1855)*
Terebellides stroemi M. Sars, 1835
Trichobranchus glacialis Malmgren, 1865*
CRUSTACEA
CIRRIPEDIA
Balanus eburneus Gould, 1841
Balanus improvisus Darwin, 1854
Chthamalus stellatus (Poli, 1795)
Euraphia depressa (Poli, 1795)
Verruca spengleri Darwin, 1854
DECAPODA
Hippolyte longirostris (Czerniavsky, 1868)
Hippolyte sapphica d'Udekem d'Acoz, 1993
Lysmata seticaudata (Risso, 1816)
Alpheus dentipes Gurin-Mneville, 1832
Athanas nitescens (Leach, 1814)
Palaemon adspersus Rathke, 1837
Palaemon elegans Rathke, 1837
Palaemon serratus (Pennant, 1777)
Crangon crangon (Linnaeus, 1758)
Philocheras fasciatus (Risso, 1816)
Philocheras trispinosus (Hailstone, 1835)
Processa edulis (Risso, 1816)
Homarus gammarus (Linnaeus, 1758)
Astacus leptodactylus Eschscholtz, 1823
Astacus pachypus Rathke, 1837
Upogebia pusilla (Petagna, 1792)
Callianassa truncata (Giard & Bonnier, 1890)
Pestarella candida (Olivi, 1792)
Clibanarius erythropus (Latreille, 1818)
Diogenes pugilator (Roux, 1829)
Pisidia longimana (Risso, 1816)
Macropodia longirostris (Fabricius, 1775)
Macropodia rostrata (Linnaeus, 1761)
Callinectes sapidus Rathbun, 1896
Carcinus aestuarii Nardo, 1847
Pirimela denticulata (Montagu, 1808)
Polybius depurator (Linnaeus, 1758)
Polybius navigator (Herbst, 1794)
Portumnus latipes (Pennant, 1777)
Sirpus ponticus Vereshchaka, 1989
Potamon potamios (Olivier, 1804)
Eriphia verrucosa (Forskl, 1785)
Pilumnus hirtellus (Linnaeus, 1761)
Rhithropanopeus harrisii (Gould, 1841)
Xantho poressa (Olivi, 1792)
Brachynotus sexdentatus (Risso, 1827)
Pachygrapsus marmoratus (Fabricius, 1787)
Planes minutus (Linnaeus, 1758)
MYSIDA
Siriella jaltensis jaltensis Czerniavsky, 1868
Gastrosaccus sanctus (Van Beneden, 1861)
Leptomysis lingvura (G. O. Sars, 1866)
Leptomysis truncata (Heller, 1863)
Acanthomysis strauchi (Czerniavsky, 1882)
Hemimysis anomala G.O. Sars, 1907
Hemimysis lamornae (Couch, 1856)
Hemimysis serrata Bacescu, 1938
Diamysis bahirensis (G.O. Sars, 1877)
Diamysis pengoi (Czerniavsky, 1882)
Diamysis mecznikovi (Czerniavsky, 1882)
Limnomysis benedeni Czerniavsky, 1882
Mesopodopsis slabberi (van Beneden, 1861)
Katamysis warpachowsky G.O. Sars, 1877
Paramysis agigensis Bacescu, 1940
Paramysis arenosa (G.O. Sars, 1877)
Paramysis baeri Czerniavsky, 1882
Paramysis bakuensis G.O. Sars, 1895
Paramysis kessleri (Grimm, 1875)
Paramysis kosswigi Bacescu, 1948
Paramysis kroyeri (Czerniavsky, 1882)
Paramysis lacustris tanaitica Martinov, 1924
Paramysis pontica Bacescu, 1938
Paramysis sowinskii Daneliya, 2002
Paramysis ullskyi (Czerniavsky, 1882)
CUMACEA
Schizorhamphus eudorelloides (G.O. Sars, 1894)
Schizorhamphus scabriusculus (G.O. Sars, 1894)
Volgacuma telmatophora Derzhavin, 1912
Pterocuma pectinatum (Sowinsky, 1893)
Pterocuma rostratum (G.O. Sars, 1894)
Pterocuma sowinskyi (G.O. Sars, 1894)
Pseudocuma (Pseudocuma) ciliatum G.O. Sars, 1879
Pseudocuma (Pseudocuma) longicorne (Bate, 1858)
Pseudocuma (Stenocuma) cercarioides G.O. Sars 1894
Pseudocuma (Stenocuma) laeve G.O. Sars, 1914
Pseudocuma (Stenocuma) graciloides G.O. Sars, 1894
Bodotria arenosa mediterranea (Stener, 1938)
Iphinoe maeotica (Sowinsky, 1894)
Iphinoe tenella G.O. Sars, 1878
Iphinoe elisae Bacescu, 1950
Cumopsis goodsir (Van Beneden, 1861)
Nannastacus euxinicus Bacescu, 1951
Cumella (Cumella) limicola G.O. Sars, 1879
Cumella (Cumella) pygmaea euxinica Bacescu, 1950
Eudorella truncatula (Bate, 1856)
Leucon (Epileucon) longirostris G.O. Sars, 1871
TANAIDACEA
Apseudes acutifrons G.O. Sars, 1882
Heterotanais oerstedi (Kroyer, 1842)
Leptochelia savignyi (Kroyer, 1842)
Pseudoleptochelia merginellae (Smith, 1906)
Pseudotanais borceai Bacescu, 1960
Tanais dulongii (Audouin, 1826)
ISOPODA
Limnoria tuberculata Sowinsky, 1884
Eurydice dollfusi Monod, 1930
Eurydice racovitzai Bacescu, 1949
Eurydice pontica (Czerniavsky, 1868)
Eurydice spinigera Hansen, 1890
Eurydice valkanovi Bacescu, 1949
Anilocra physodes (Linnaeus, 1758)
Mothocya taurica (Czerniavsky, 1868)
Cymodoce erythraea euxinica Bacescu, 1958
Cymodoce aff. tattersalli Torelli, 1929
Dynamene bidentatus (Adams, 1800)
Dynamene bicolor (Rathke, 1837)
Exosphaeroma pulchellum Colosi, 1921
Sphaeroma serratum (Fabricius, 1787)
Idotea balthica (Pallas, 1772)
Idotea ostroumovi Sowinsky, 1895
Synisoma capito (Rathke, 1837)
Porcellio lamellatus Budde-Lund, 1885
Tylos europaeus Arcangeli, 1938
Tylos ponticus Grebnitsky, 1874
Jaera hopeana A. Costa, 1853
Jaera nordmanni (Rathke, 1837)
Jaera sarsi Valkanov, 1936
Bopyrina ocellata (Czerniavsky, 1868)
Bopyrissa diogeni (Popov, 1927)
Parathelges racovitzai R. Codreanu, 1940
Progebiophilus euxinicus (Popov, 1929)
Ligia italica Fabricius, 1798
Halophiloscia couchii (Kinahan, 1858)
Halophiloscia pontica Radu, 1985
Elaphognathia bacescoi (Kussakin, 1969)
Gnathia oxyuraea (Lilljeborg, 1855)
AMPHIPODA
Orchomenehumilis (A. Costa, 1853)
Nannonyx goesi (Boeck, 1871)
Nannonyx propinquus Chevreux, 1911
Ampeliscadiadema (A. Costa, 1853)
Ampeliscapseudospinimana Bellan-Santini & Kaim-Malka, 1977
Bathyporeia guilliamsoniana (Bate, 1857)
Harpinia dellavallei Chevreux, 1910
Stenothoemonoculoides (Montagu, 1815)
Perioculodeslongimanus longimanus (Bate & Westwood, 1868)
Synchelidiummaculatum Stebbing, 1906
Monoculodes gibbosus Chevreux, 1888
Apherusabispinosa (Bate, 1857)
Apherusachiereghinii Giordani-Soika, 1950
Atylus guttatus (A. Costa, 1851)
Atylusmassiliensis Bellan-Santini, 1975
Biancolina algicola Della Valle, 1893
Cymadusa crassicornis (A. Costa, 1857)
Amathillina cristata G.O. Sars, 1894
Cardiophilus baeri G.O. Sars, 1896
Dikerogammarus haemobaphes (Eichwald, 1841)
Dikerogammarus villosus (Sowinsky, 1894)
Echinogammarus foxi (Schellenberg, 1928)
Echinogammarus ischnus (Stebbing, 1899)
Echinogammarus olivii (Milne-Edwards, 1830)
Echinogammarus placidus (G.O. Sars, 1896)
Echinogammarus warpachowskyi (G.O. Sars, 1894)
Ericthonius punctatus (Bate, 1857)
Ericthoniusdifformis Milne-Edwards, 1830
Euxinia maeoticus (Sowinsky 1894)
Euxinia sarsi (Sowinsky, 1898)
Euxinia weidemanni (G.O. Sars, 1896)
Gammarellusangulosus (Rathke, 1843)
Gammarus aequicauda (Martynov, 1931)
Gammarus crinicornis Stock, 1966
Gammarus duebeni Liljeborg, 1852
Gammarus insensibilis Stock, 1966
Gammarus pulex (Linnaeus, 1758)
Gammarus subtypicus Stock, 1966
Gammarus zaddachi Sexton, 1912
Gammaruslocusta (Linnaeus, 1758)
Gammarusmarinus Leach, 1815
Iphigenella andrussowi (G.O. Sars, 1896)
Iphigenella shablensis (Carausu, 1943)
Megaluropusagilis Hoeck, 1889
Melitapalmata (Montagu, 1804)
Obesogammarus crassus (G.O. Sars, 1894)
Obesogammarus obesus (G.O. Sars, 1894)
Pontogammarus robustoides (G.O. Sars, 1894)
Stenogammarus carausui Derzhavin & Pjatakova 1962
Stenogammarus compressus (G.O. Sars, 1894)
Stenogammarus macrurus (G.O. Sars, 1894)
Stenogammarus similis (G.O. Sars, 1894)
Uroniphargoides spinicaudatus (Carausu, 1943)
Yogmelina pusilla (G.O. Sars, 1896)
Dexaminespinosa (Montagu, 1813)
Tritaeta gibbosa (Bate, 1862)
Orchestia cavimana Heller, 1865
Orchestia mediterranea A. Costa, 1853
Orchestia stephenseni Cecchini, 1928
Orchestiagammarellus (Pallas, 1766)
Orchestiamontagui Audouin, 1826
Parhyale aquilina (A. Costa, 1857)
Talitrussaltator (Montagu, 1808)
Talorchestia brito Stebbing, 1891
Talorchestia deshayesi (Audouin, 1826)
Platorchestia platensis Kroyer, 1845
Hyale crassipes (Heller, 1866)
Hyale dollfusi Chevreux, 1911
Hyale schmidti (Heller, 1866)
Hyaleperieri (Lucas, 1849)
Hyalepontica Rathke, 1837
Hyaleprevosti (Milne-Edwards, 1830)
Microdeutopus algicola Della Valle, 1893
Microdeutopusanomalus (Rathke, 1843)
Microdeutopusdamnoniensis (Bate, 1856)
Microdeutopusgryllotalpa A. Costa, 1853
Microdeutopusversiculatus (Bate, 1856)
Microprotopus longimanus Chevreux, 1887
Megamphopuscornutus Norman, 1869
Leptocheiruspilosus Zaddach, 1844
Ampithoegammaroides (Bate, 1856)
Ampithoehelleri Karaman, 1975
Ampithoeramondi Audouin, 1826
Atylus guttatus (A. Costa, 1851)
Atylusmassiliensis Bellan-Santini, 1975
Biancolina algicola Della Valle, 1893
Cymadusa crassicornis (A. Costa, 1857)
Jassa marmorata (Holmes, 1903)
Jassaocia (Bate, 1862)
Corophium acherusicum A. Costa, 1851
Corophium maeoticum Sowinsky, 1898
Corophium nobile G.O. Sars, 1895
Corophium orientale Schellenberg, 1928
Corophium robustum G.O. Sars, 1895
Corophium sowinskyi Martynov, 1924
Corophiumbonnellii (Milne-Edwards, 1830)
Corophiumcrassicorne Bruzelius, 1859
Corophiumcurvispinum G.O. Sars, 1895
Corophiumruncicorne Della Valle, 1893
Ericthonius difformis (Dana, 1855)
Ericthonius punctatus (Bate, 1857)
Siphonoecetes dellavallei Stebbing, 1899
Chelura terebrans Philippi, 1839
Caprellaacanthifera Leach, 1814
Caprella acanthifera discrepans Carausu, 1941
Caprella liparotensis Haller, 1879
Caprella rapax Mayer, 1890
Caprelladanilevskii Czerniavski, 1868
Caprellamitis Mayer, 1890
Pseudoprotellaphasma (Montagu, 1804)
Phtisicamarina Slabber, 1749
MOLLUSCA
POLYPLACOPHORA
Lepidochitona caprearum Scacchi, 1836
Lepidochitona cinerea Linnaeus, 1767
GASTROPODA
Patella caerulea Linnaeus, 1758
Calliostomagranulatum (Von Born, 1778)
Gibbulaadansonii adansonii (Payraudeau, 1826)
Gibbulaalbida (Gmelin, 1791)
Gibulla divaricata (Linnaeus, 1758)
Tricolia pullus pullus (Linnaeus, 1758)
Bittium reticulatum (da Costa, 1778)
Bittium submamillatum (de Rayneval & Ponzi, 1854)
Cerithiopsis minima (Brusina, 1865)
Cerithiopsis tubercularis (Montagu, 1803)
Marshallora adversa (Montagu, 1803)
Melarhaphe neritoides (Linnaeus, 1758)
Rissoa lilacina Rcluz, 1843
Rissoa membranacea (Adams J., 1800)
Rissoa parva (da Costa, 1778)
Rissoasplendida Eichwald, 1830
Caecumtrachea (Montagu, 1803)
Setia valvatoides (Milaschewitsch, 1909)
Rudolphosetia turriculata (Monterosato, 1884)
Hydrobia acuta (Draparnaud, 1805)
Hydrobiaventrosa (Montagu, 1803)
Ventrosia maritima (Milaschewitsch, 1916)
Bella nebula (Montagu, 1803)
Truncatella subcylindrica (Linnaeus, 1767)
Calyptrea chinensis (Linnaeus, 1758)
Epitoniumcommune (Lamarck, 1822)
Trophonopsis breviatus (Jeffreys, 1882)
Nassariusincrassatus (Strm, 1768)
Nassarius nitidus (Jeffreys, 1867)
Nassariusreticulatus (Linnaeus, 1758)
Cyclope neritea (Linnaeus, 1758)
Rapanavenosa (Valenciennes, 1846)
Omalogyra atomus (Philippi, 1841)
Chrysallida brusinai (Cossmann, 1921)
Chrysallida emaciata (Brusina, 1866)
Chrysallida indistincta (Montagu, 1808)
Chrysallida interstincta (Adams J., 1797)
Eulimella aciculata (Phillippi, 1836)
Odostomia erjaveciana Brusina, 1869
Odostomia eulimoides Hanley, 1844
Odostomia scalaris MacGillivray, 1843
Odostomia plicata (Montagu, 1803)
Turbonilla delicata (Monterosato, 1874)
Ebala pointeli (de Folin, 1868)
Retusa mammillata (Philippi, 1836)
Retusa piriformis Monterosato, 1878
Retusatruncatula (Bruguire, 1792)
Cylichninaumbilicata (Montagu, 1803)
Corambe obscura (Verril, 1870)
Tergipes tergipes (Forskl, 1775)
Tenellia adspersa Nordmann, 1845
Embletonia pulchra (Alder & Hancock 1844)
Myosotella myosotis (Draparnaud, 1801)
BIVALVIA
Anadara inaequivalvis (Bruguire, 1789)
Striarcalactea (Linnaeus, 1758)
Mytilusgalloprovincialis Lamarck, 1819
Mytilasterlineatus (Gmelin, 1791)
Modiolusadriaticus (Lamarck, 1819)
Modiolusbarbatus (Linnaeus, 1758)
Modiolulaphaseolina (Philippi, 1844)
Pinnarudis Linnaeus, 1758
Chlamysflexuosa (Poli, 1795)
Chlamys glabra (Linnaeus, 1758)
Ostreaedulis Linnaeus, 1758
Loripeslacteus (Linnaeus, 1758)
Lucinelladivaricata (Linnaeus, 1758)
Kelliasuborbicularis (Montagu, 1803)
Mysellabidentata (Montagu, 1803)
Acanthocardiapaucicostata (Sowerby G.B. II, 1841)
Acanthocardiatuberculata (Linnaeus, 1758)
Parvicardiumexiguum (Gmelin, 1791)
Plagiocardiumpapillosum (Poli, 1795)
Cerastodermaglaucum (Poiret, 1789)
Mactrastultorum (Linnaeus, 1758)
Spisulasolida (Linnaeus, 1758)
Spisulasubtruncata (da Costa, 1778)
Donacilla cornea (Poli, 1791)
Solenmarginatus Pulteney, 1799
Tellina distorta Poli, 1791
Telinna donacina (Linnaeus, 1758)
Tellinafabula Gmelin, 1791
Tellinatenuis da Costa, 1778
Gastranafragilis (Linnaeus, 1758)
Donaxtrunculus Linnaeus, 1758
Donaxvenustus Poli, 1795
Abraalba (Wood W., 1802)
Abranitida (O.F. Mller, 1776)
Abra ovata (Philippi, 1836)
Abraprismatica (Montagu, 1808)
Venuscasina Linnaeus, 1758
Chameleagallina (Linnaeus, 1758)
Gouldiaminima (Montagu, 1803)
Pitarrudis (Poli, 1795)
Paphiaaurea (Gmelin, 1791)
Myaarenaria Linnaeus, 1758
Lentidiummediterraneum (O.G. Costa, 1829)
Pholasdactylus Linnaeus, 1758
Barneacandida (Linnaeus, 1758)
* Species (Polychaeta) not found in the Black Sea but reported from the Bosphorus strait.